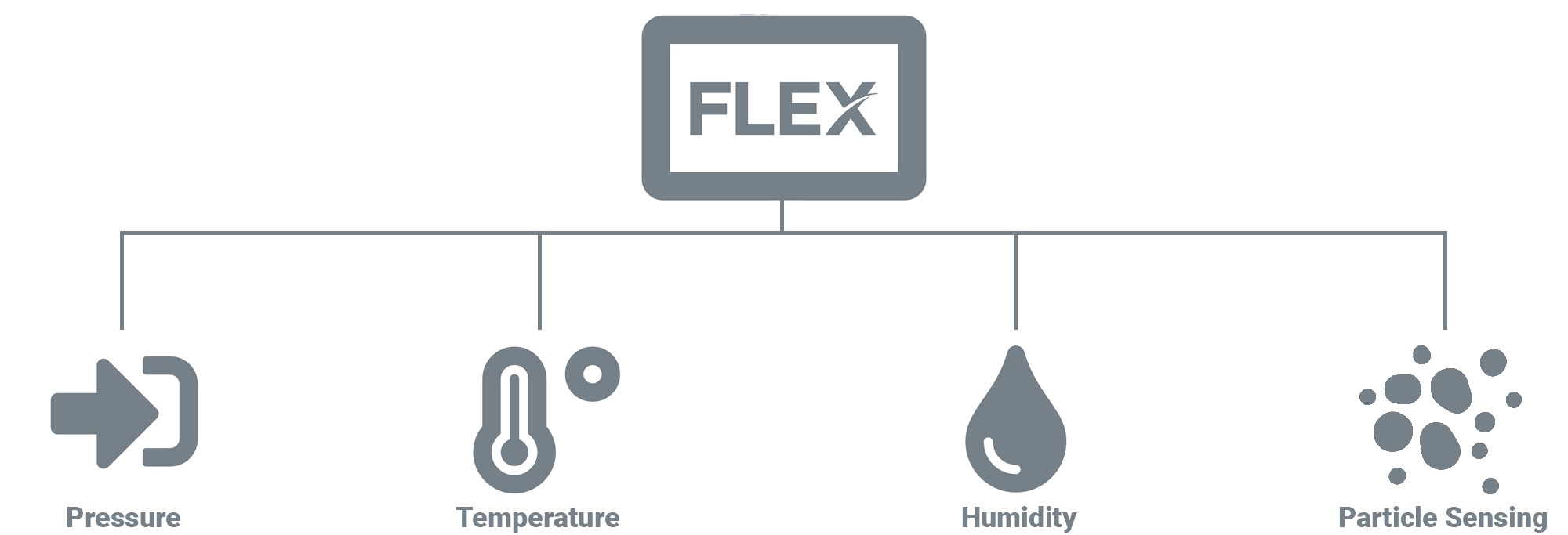
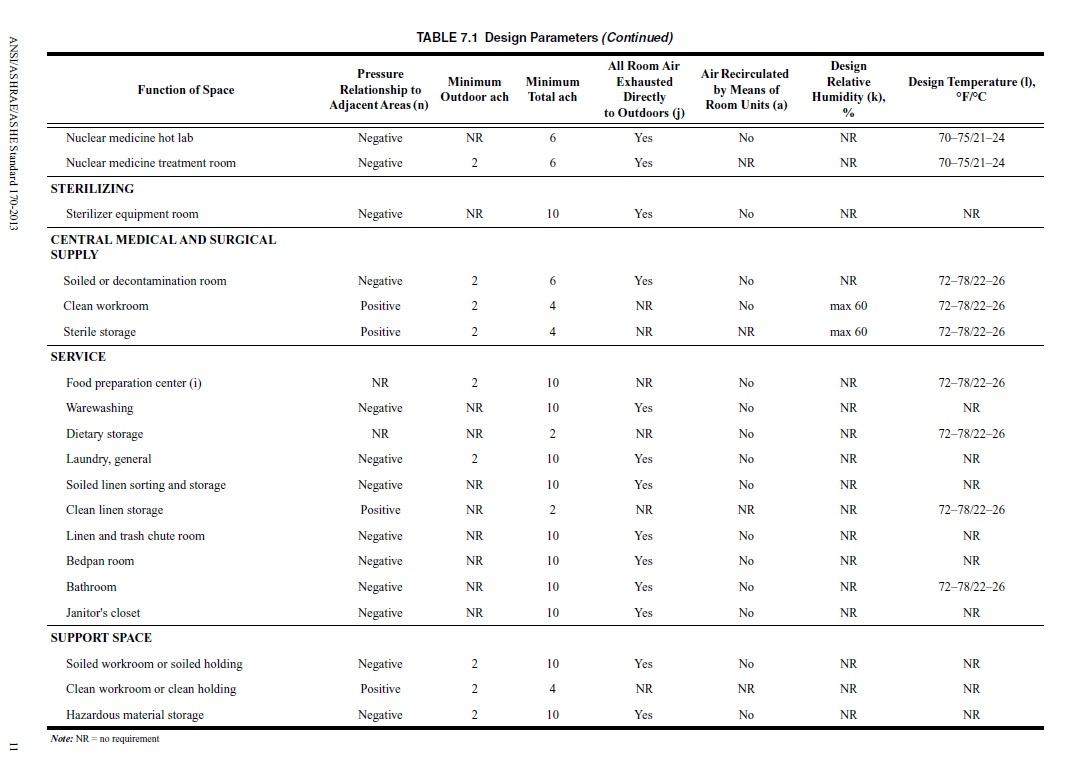
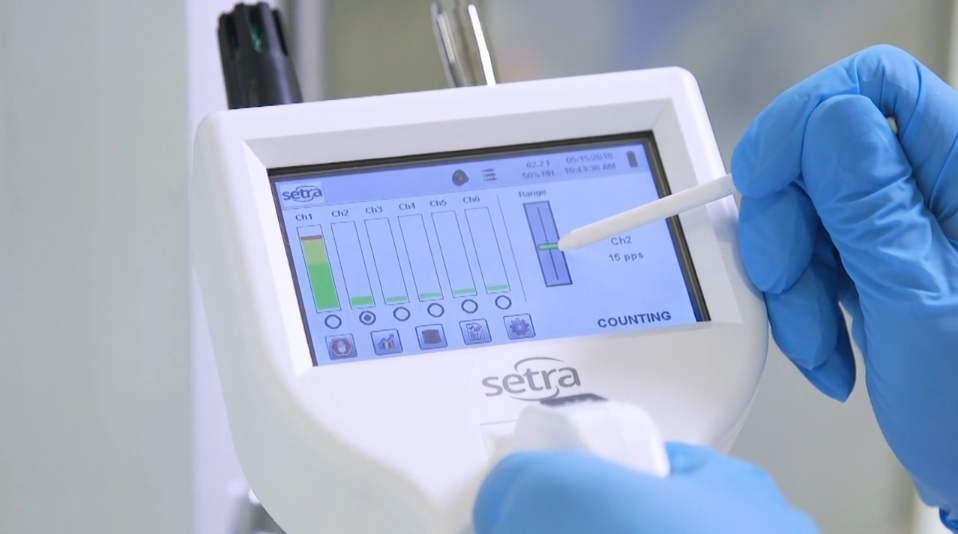
Requirements and regulations are becoming ever more stringent for critical environments, making finding the right products for a space increasingly difficult. Not only must the specs of the product be considered, but the supplier must be taken into account as well. Shopping around with the intent to purchase from a variety of vendors takes a significant amount of time and effort. In addition to wasted time and effort, buying from multiple vendors comes associated with a number of risks and drawbacks, including:
- Incompatible hardware
- Incompatible software
- Minimal or disjointed tech support
- Inability to integrate products
- Installation and operational issues