- Website Pages
- Products
Easy Access to Data · Reduced Human Errors · Data Tracking Capabilities
When patient safety and quality of compounded medications is critical, trust Setra CEMS to ensure environmental parameters stay within limit.
Hospital pharmacies are unique because of the critical role they play in maintaining patient health and safety. Pharmacies that perform compounded sterile preparations (CSPs), in particular, must meet specific clean room standards to ensure sterile conditions are maintained. While there are many established environmnetal requirements in these spaces, some pharmacy managers are choosing to go the extra mile in exchange for added peace of mind.
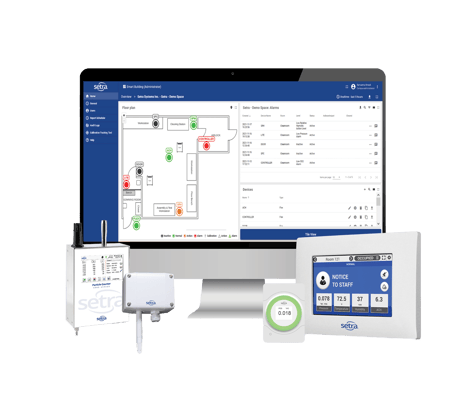
Setra CEMS™ solution paired with our sensing hardware enable you to gain real-time insights into all your locations. With our cloud-based monitoring system, you gain data visualization and actionable insights as well as automated reporting, quality integrity, and risk mitigation.
- Full life-cycle guidance from industry experts in hardware and software implementation
- Not limited to Setra devices - easily connect 3rd party sensors
- Eliminate manual data logging and reporting
- Automatically monitor data to ensure the desired airflow, temperature, and humidity levels are achieved for regulatory compliance.
The Setra CEMS solution is designed to solve your most important pharmacy safety problems. The solution combines start-of-the-art sensor hardware with cloud-based monitoring software and digital compliance reporting. The data turns into actionable insights that drive quality control, staff productivity, and regulatory compliance.

Patient/customer safety will always be the #1 priority when it comes to storing and compounding medications. From compounding medication, to the time of sale and the point of administering the product to patients, we need to continually monitor the fabrication, storage and transfer of these products both inside and outside of controlled areas. For medications that are exposed to the air, sterilization is key for maintaining proper quality control and the integrity of the product.
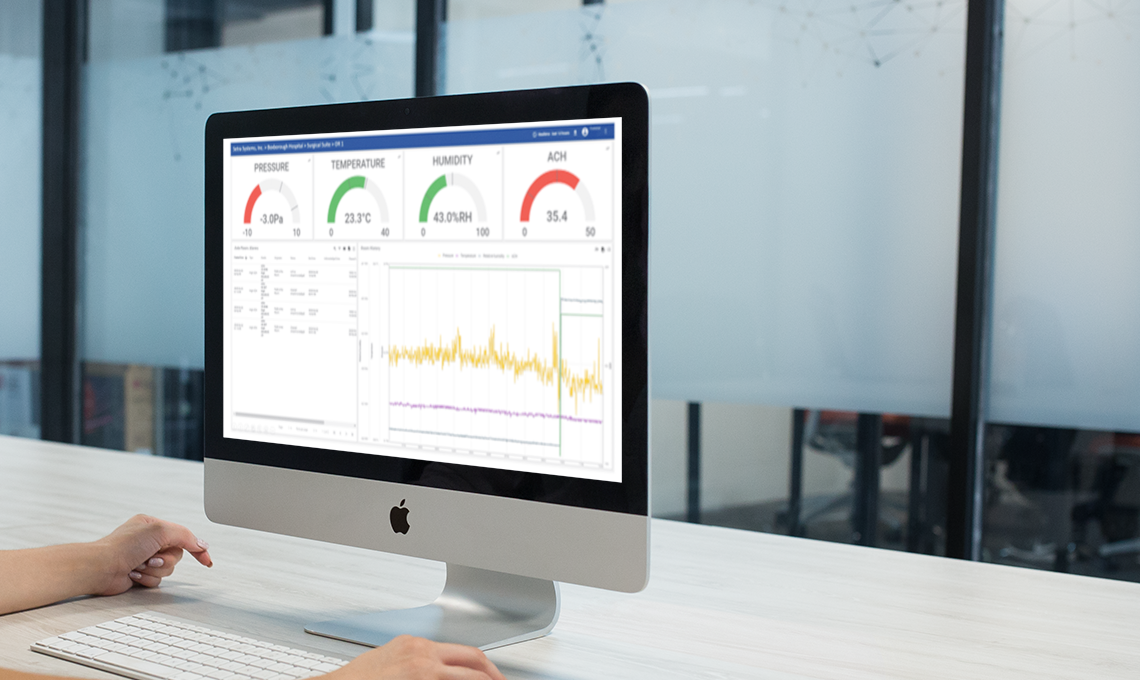
Medication, vaccines, blood and tissues must be stored in specified temperature and relative humidity environments, these environments require continuous monitoring! Setra’s Continuous Environmental Monitoring System and Software allows you to seamlessly monitoring critical areas and appliances inside your pharmacy space. within pharmacy refrigerators and freezers and within blood and tissue banks.
- Measure and monitor: Temp, %rh, Differential Pressure, CO2 to the highest accuracy
- Real-time data monitoring and alerts
- FDA CFR 21 Part 11 compliant software
Setra’s CEMS helping users monitor their GxP compliant applications, looking into the critical quality attributes and monitoring critical process parameters, helping focus on patient safety, product quality and data integrity and compliant to EudraLex Annex 11 and FDA 21 CFR Part 11.